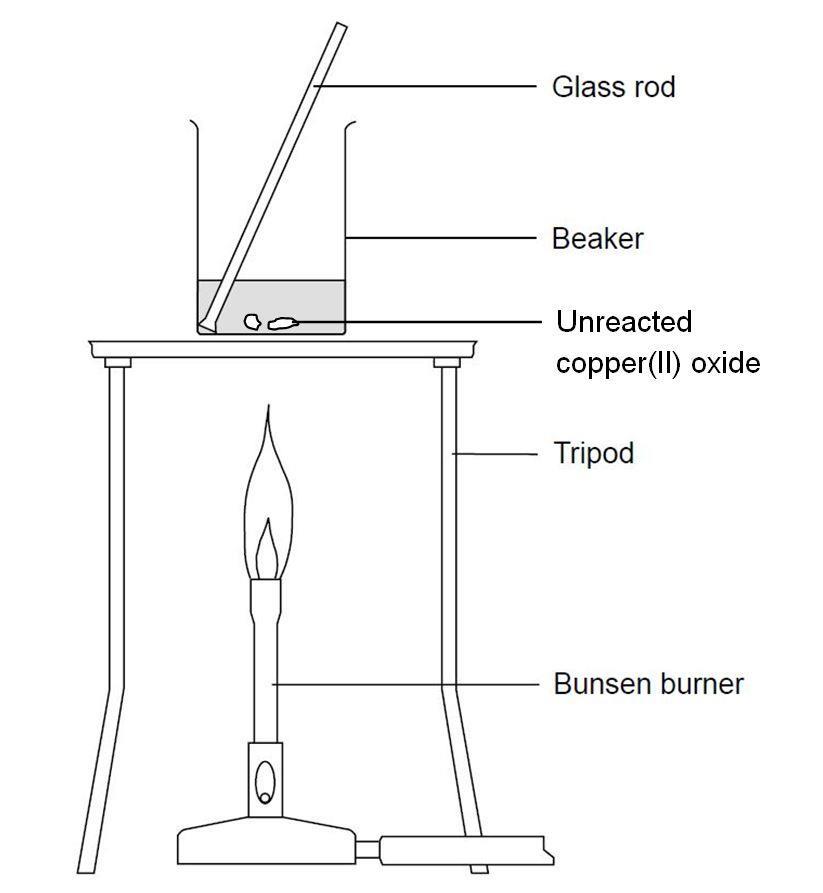
Copper Oxide Reacts With Sulphuric Acid . When the acid is hot enough (just before it starts to boil), use a. The copper takes the [o] and. copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. copper oxide reacts with sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. apparatus for heating copper (ii) oxide and dilute sulfuric acid. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. learn how copper oxide and sulphuric acid react to form copper sulphate and water. learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid.
from edu.rsc.org
mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. The copper takes the [o] and. learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid. apparatus for heating copper (ii) oxide and dilute sulfuric acid. you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. learn how copper oxide and sulphuric acid react to form copper sulphate and water. When the acid is hot enough (just before it starts to boil), use a. copper oxide reacts with sulfuric acid.
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education
Copper Oxide Reacts With Sulphuric Acid apparatus for heating copper (ii) oxide and dilute sulfuric acid. copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. The copper takes the [o] and. learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. copper oxide reacts with sulfuric acid. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. apparatus for heating copper (ii) oxide and dilute sulfuric acid. learn how copper oxide and sulphuric acid react to form copper sulphate and water. When the acid is hot enough (just before it starts to boil), use a.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Oxide Reacts With Sulphuric Acid apparatus for heating copper (ii) oxide and dilute sulfuric acid. The copper takes the [o] and. you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. copper oxide reacts with sulfuric acid. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and. Copper Oxide Reacts With Sulphuric Acid.
From www.numerade.com
SOLVED The reaction between solid copper(Il) oxide (also called cupric oxide) and aqueous Copper Oxide Reacts With Sulphuric Acid copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. The copper takes the [o] and. apparatus for heating copper (ii) oxide and dilute sulfuric acid. copper oxide reacts with sulfuric acid. learn how copper oxide and sulphuric acid react to form copper sulphate and water.. Copper Oxide Reacts With Sulphuric Acid.
From www.alamy.com
Beaker being used to add sulphuric acid to copper (II) oxide (black) at the bottom of a test Copper Oxide Reacts With Sulphuric Acid you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. copper oxide reacts with sulfuric acid. apparatus for heating copper (ii) oxide and dilute sulfuric acid. When the acid is hot enough (just before it starts to boil), use a. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal. Copper Oxide Reacts With Sulphuric Acid.
From www.alamy.com
copper oxide and sulphuric acid form copper sulphate solution which is filtered to remove Copper Oxide Reacts With Sulphuric Acid apparatus for heating copper (ii) oxide and dilute sulfuric acid. copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid. you can imagine concentrated sulfuric acid as an. Copper Oxide Reacts With Sulphuric Acid.
From www.youtube.com
Reaction of Copper Oxide With Hydrochloric Acid YouTube Copper Oxide Reacts With Sulphuric Acid copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. apparatus for heating copper (ii) oxide and dilute sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. . Copper Oxide Reacts With Sulphuric Acid.
From www.youtube.com
Reaction of copper oxide and sulfuric acid YouTube Copper Oxide Reacts With Sulphuric Acid learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid. The copper takes the [o] and. copper oxide reacts with sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. . Copper Oxide Reacts With Sulphuric Acid.
From www.youtube.com
Cu + H2SO4 (Copper + Sulfuric acid) YouTube Copper Oxide Reacts With Sulphuric Acid When the acid is hot enough (just before it starts to boil), use a. learn how copper oxide and sulphuric acid react to form copper sulphate and water. copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. you can imagine concentrated sulfuric acid as an oxidising. Copper Oxide Reacts With Sulphuric Acid.
From www.numerade.com
SOLVED We can use copper oxide in the laboratory to make copper sulfate by reacting it with Copper Oxide Reacts With Sulphuric Acid learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid. learn how copper oxide and sulphuric acid react to form copper sulphate and water. apparatus for heating copper (ii) oxide and dilute sulfuric acid. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide. Copper Oxide Reacts With Sulphuric Acid.
From www.sciencephoto.com
Copper (II) oxide reacting with sulphuric acid Stock Image C052/5207 Science Photo Library Copper Oxide Reacts With Sulphuric Acid The copper takes the [o] and. When the acid is hot enough (just before it starts to boil), use a. copper oxide reacts with sulfuric acid. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. learn how to write the balanced equation for. Copper Oxide Reacts With Sulphuric Acid.
From www.alamy.com
Concentrated sulphuric acid turns blue copper sulphate crystals into white anhydrous copper Copper Oxide Reacts With Sulphuric Acid learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid. learn how copper oxide and sulphuric acid react to form copper sulphate and water. The copper takes the [o] and. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a. Copper Oxide Reacts With Sulphuric Acid.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Oxide Reacts With Sulphuric Acid cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. When. Copper Oxide Reacts With Sulphuric Acid.
From tanianewsbridges.blogspot.com
Copper Oxide Sulfuric Acid Copper Oxide Reacts With Sulphuric Acid copper oxide reacts with sulfuric acid. When the acid is hot enough (just before it starts to boil), use a. learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric acid. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide. Copper Oxide Reacts With Sulphuric Acid.
From www.alamy.com
Two copper oxide reactions. Heating copper oxide with dilute sulphuric acid results in a blue Copper Oxide Reacts With Sulphuric Acid cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. learn how copper oxide and sulphuric acid react to form copper sulphate and water. copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated.. Copper Oxide Reacts With Sulphuric Acid.
From daisy-yersblogchoi.blogspot.com
Word Equation for Copper Oxide and Sulfuric Acid Copper Oxide Reacts With Sulphuric Acid mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. When the acid is hot enough (just before it starts to boil), use a. learn how copper oxide and sulphuric acid react to form copper sulphate and water. copper(ii) oxide reacts. Copper Oxide Reacts With Sulphuric Acid.
From pixels.com
Copper Oxide And Sulphuric Acid Photograph by Martyn F. Chillmaid/science Photo Library Pixels Copper Oxide Reacts With Sulphuric Acid The copper takes the [o] and. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. copper oxide. Copper Oxide Reacts With Sulphuric Acid.
From edu.rsc.org
Reacting copper(II) oxide with sulfuric acid Experiment RSC Education Copper Oxide Reacts With Sulphuric Acid learn how copper oxide and sulphuric acid react to form copper sulphate and water. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. you can imagine concentrated sulfuric acid as an oxidising agent which supplies [o]. The copper takes the. Copper Oxide Reacts With Sulphuric Acid.
From www.numerade.com
SOLVED I am required to find the molecular equation, ionic equation, and net ionic equation for Copper Oxide Reacts With Sulphuric Acid copper oxide reacts with sulfuric acid. cuo + h2so4 = cuo4s + h2o is a double displacement (metathesis) reaction where one mole of copper(ii) oxide [cuo] and one mole. apparatus for heating copper (ii) oxide and dilute sulfuric acid. learn how to write the balanced equation for the double replacement reaction between copper oxide and sulfuric. Copper Oxide Reacts With Sulphuric Acid.
From www.toppr.com
Sulphuric acid reacts with cupric oxide to give Copper Oxide Reacts With Sulphuric Acid copper(ii) oxide reacts with mineral acids such as hydrochloric acid, sulfuric acid, and nitric acid to give the corresponding hydrated. mixing copper oxide and sulphuric acid is an experiment involving an insoluble metal oxide which is reacted with a dilute acid to form a soluble salt. learn how copper oxide and sulphuric acid react to form copper. Copper Oxide Reacts With Sulphuric Acid.